Hearing impairment after subarachnoid hemorrhage
By: Karen Holzberger, President & CEO of SpinTech MRI
Author(s): Nicci Campbell1 , Carl Verschuur1 , Sophie Mitchell2 , Orlaith McCaffrey1 , Lewis Deane1 , Hannah Taylor1 , Rory Smith1 , Lesley Foulkes3 , James Glazier2 , Angela Darekar4 , Mark E. Haacke5 , Diederik Bulters3,# & Ian Galea2,3,#
Journal: Annals of Clinical and Translational Neurology
Published: 2019
Read Full Paper: https://onlinelibrary.wiley.com/doi/full/10.1002/acn3.714
Abstract
Background
Subarachnoid hemorrhage (SAH) survivors experience significant neurological disability, some of which is under‐recognized by neurovascular clinical teams. We set out to objectively determine the occurrence of hearing impairment after SAH, characterize its peripheral and/or central origin, and investigate likely pathological correlates.
Method
In a case‐control study (n = 41), participants were asked about new onset hearing difficulty 3 months post‐SAH, compared with pre‐SAH. Formal audiological assessment included otoscopy, pure tone audiometry, a questionnaire identifying symptoms of peripheral hearing loss and/or auditory processing disorder, and a test of speech understanding in noise. A separate cohort (n = 21) underwent quantitative susceptibility mapping (QSM) of the auditory cortex 6 months after SAH, for correlation with hearing difficulty.
Results
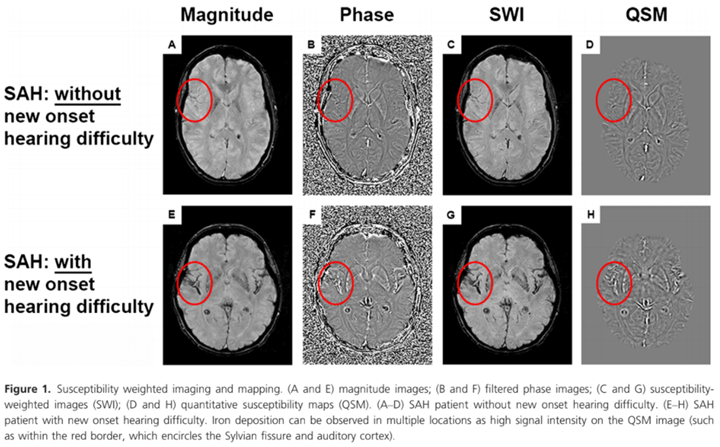
Twenty three percent of SAH patients reported hearing difficulty that was new in onset post‐SAH. SAH patients had poorer pure tone thresholds compared to controls. The proportion of patients with peripheral hearing loss as defined by the World Health Organization and British Audiological Society was however not increased, compared to controls. All SAH patients experienced symptoms of auditory processing disorder post‐SAH, with speech‐in‐noise test scores significantly worse versus controls. Iron deposition in the auditory cortex was higher in patients reporting hearing difficulty versus those who did not.
Conclusions
This study firmly establishes hearing impairment as a frequent clinical feature after SAH. It primarily consists of an auditory processing disorder, mechanistically linked to iron deposition in the auditory cortex. Neurovascular teams should inquire about hearing, and refer SAH patients for audiological assessment and management.

